Comparative performances of plastic/frozen/thick sections
Why we recommend expoy resin sections for anti-hapten immunocytochemistry
Users often ask why they cannot use methods other than resins. The answer is performance. The literature on immunocytochemical amino acid detection is unequivocal. Resin sections surpass all other histological methods in optical resolution, repeatability, and sensitivity. The technology of resin sectioning is mature and protocols well-developed.
Resin sections present a superior probe surface
The surface variation in etched epoxy resin sections is extremely low compared to paraffin, frozen, or thick tissue-slice preparations. This means that the optical surface for signal detection and measurement is superior to any other method by 1-2 orders of magnitude. A rough surface generates an unsatisfactory signal due to irregular superposition effects.
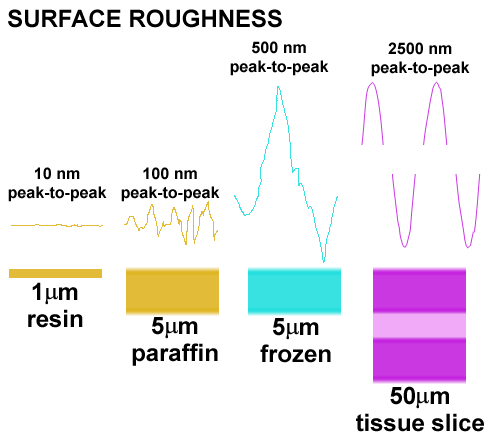
HPI on resin sections is independent of section thickness
The signals generated in HPI arise from IgGs bound to the surface of the section. This means that HPI is quantitative in the same way as an immunoblot and makes the probe surface a critical parameter. It also means that ultrathin serial sections can be used optically and that repeated measures give identical results even if section thickness varies. Only resin sections have sufficient dimensional stability and superior surface characteristics to ensure high performance.
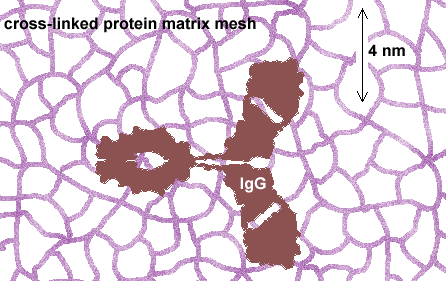
IgGs do not penetrate the fixed protein matrix
Quantitative or even statistically meaningful qualitative detection of haptens requires that they be captured in a cross-linking conjugation that "fixes" the free hapten to the protein matrix of the cell. This process also cross-links the matrix, producing a mesh with a pore size of about 2 nm. The dimensions of IgGs are about 4 x 7 x 7 nm with a forked shape. IgGs cannot physically penetrate the fixed protein mesh unless it is substantially fractured by freeze-thaw or protease procedures. Unfortunately, such fracturing forms uneven, unpredictable modes of penetration and also renders the section surface rough. Resin sections are superior to all other media in this regard.
IgGs do not readily penetrate cell membranes
Fixed cell membranes retain substantial structural integrity, even after aggressive lipid extraction in tissue processing (e.g. phospatidylinositol appears very resistant to extraction). This is irrelevant with resin sections and only marginally of concern in paraffin material, but a serious defect of frozen sections and tissue slices. Haptens inside cells can only be detected if the IgG enters the protein matrix (see above), which requires that the cells be cut open, broken open or patches of membrane removed by detergent exposure. Again, the penetration is uneven and unpredictable, the section surface is further distorted, and the penetration of the protein matrix must still be faced.
IgGs do not penetrate the hapten-IgG "prozone"
Anti-hapten IgGs are selective for micromolecules present at 10 to 10,000 x higher concentrations than macromolecules. When such haptens bind IgGs, they form prozone-like matrices that become poorly penetrable by subsequent diffusing IgGs. This is a serious problem with hapten detection in thick sections, but is completely overcome by the use of thin resin sections.
Performance Characteristics for Detecting Micromolecules with Different Tissue Sampling Methods
| Methods | Signal Strength | Quantitative Detection | IgG penetration | Glutaraldehyde tolerance3 |
|---|---|---|---|---|
| Resin Section | Maximal and constant(50-1000 nm) | Yes | Constant1 | Absolute |
| Frozen Sections | Variable | Rare | Variable (few um; hapten concentration dependent2 | Moderate (impedes IgG penetration) |
| Vibratome Sections | Variable | No | Variable (hapten concentration dependent2) | Low (impedes IgG penetration) |
| Whole Mounts | Highly Variable | No | Variable (hapten concentration-dependent2) | Low (impedes IgG penetration) |
| Tissue Culture | Variable | Variable | Variable (hapten concentration dependent2) | Variable (hapten dependent) |
1 IgG binding to etched thin sections follows the physics of two-phase partitioning (liquid to surface) and is constant with section thickness for most detection techniques. The precision of sectioning does not impact either the magnitude of a signal or the precision of successive signals.
2 Penetration of an IgG is a function of the molecular mesh through with it must weave. In general, glutaraldehyde creates a mesh much smaller than the 10 nm radius of gyration of a conventional 150Kd IgG. This is exacerbated when one attempts to detect a hapten whose concentration may enter the millimolar range. In this case, the first IgGs to bind to the outer layer of hapten creates a massive barrier to further penetration analogous to the prozone effect. n this case it is not so much the fixative (i.e. the linker) as the concentration of the hapten itself that creates is variable barrier.
3 Many antibodies designed to detect small molecules are specifically produced to recognize amino acids or similar targets stoichiometrically trapped by glutaraldehyde: a bifunctional reagent. This renders subsequent immunocytochemistry capable of quantitative comparisons of amino acid levels across cells, tissues and experimental preparations. Paraformaldehyde fixation alone cannot be used for such studies as it is a monofunctional reagent and the N-formyl amino acids arising from paraformaldehyde fixation are extremely soluble, diffusing rapidly out of tissue during histological processing. However, a slow secondary reaction involving N-formyl amino acids and immobile matrix amines (and perhaps other groups) can result in the formation of short "methine" bridges that stabilize a small fraction of the target. This secondary reaction is temperature and time dependent and its efficacy further impacted strongly by the hapten, pH, etc. Thus paraformaldehyde fixation is not desirable for quantitative measures of intracellular targets.
© 2000 Signature Immunologics, Inc.
Copying and distribution:
This Technical Note may be copied and distributed for educational and non-profit
purposes as long as acknowledgement of Signature Immunologics' copyright is included
in every instance of use. It may not be sold or distributed for commercial gain.