December 2007 Archives
1-amino-4-guanidinobutane (AGB)
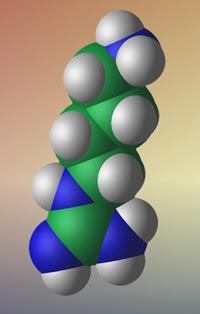
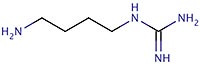
| COMMON NAMES: | Agmatine2+, 1-amino-4-guanidinobutane (AGB) |
| IUPAC NAME: | 4-Aminobutyliminomethanediamine |
| FORMULA: | C5H14N4 |
| SMILES STRING: | NCCCCNC(N)=N |
| MW: | 130.192 |
| pK1: | 12.5 |
| pK2: | 9 |
| CAS: | 2482-00-0 |
| LINKS: |
HMDB KEGG |
| Notes: AGB is commercially available as a sulfate salt from Sigma-Aldrich at 97-99% purity. The major contaminant is usually arginine. AGB is a divalent cation at physiological pH, but the charges are at opposite ends of the molecule: +CCCC+ | |
About AGB / Agmatine
(from Marc RE, M Kalloniatis, and BW Jones. 2005 Excitation mapping with the organic cation AGB2+. Vision Research, 45: 3454-3468)
Guanidinium ions and guanidinium analogues are a well-established family of organic channel permeant cations (Balasubramanian, Lynch, & Barry, 1995; Dwyer, Adams, & Hille, 1980; Larramendi, Lorente de No, & Vidal, 1956; Tasaki, Watanabe, & Singer, 1966). Their permeation is based on a very compact, resonance-stabilized cation: C+(NH2)3. In the case of R-C+(NH2)2 derivatives, efficient aldehyde trapping and hapten conjugation can be achieved if the R group contains reactive amino groups. Fig. 1 illustrates the atomic dimensions of hydrated sodium ions (Datta & Iyengar, 1991), the guanidinium cation (GU), 1-amino-4-guanidobutane (AGB or agmatine, the natural decarboxylation product of arginine), and L-2-amino-5-guanidino-pentanoate (ARG, arginine itself). At physiological pH, AGB is a divalent cation due to its separately protonated guanidine and amino groups, while arginine is a net monovalent cation due to its additional carboxylate group. AGB likely behaves as a monovalent species in terms of initial microscopic channel interactions with the guanidinium head, but a divalent species in terms of macroscopic charge transfer. AGB is produced at extremely low levels in the mammalian nervous system and non-quantitative fluorescence imaging suggests that most of it is associated with very sparse neuronal terminals in cortex and spinal cord (Fairbanks et al., 2000). Indeed, there is a large literature on agmatine (AGB) as an endogenous imidazole receptor agonist (e.g., Raasch, Schafer, Chun, & Dominiak, 2001) and anti-inflammatory agent (Fairbanks et al., 2000), but the levels associated with these functions are clearly far below the millimolar values used in our flux experiments (see discussion in Marc, 1999b). On theoretical grounds at least, aldehyde-trapped intracellular guanidines should be quantitative reporters of time-integrated neuronal cation currents. This dependence on and compatibility with glutaraldehyde cross-linking in particular means that guanidine reporters could potentially provide high spatial resolution. If guanidine fluxes are predominantly coupled to signaling processes through neuronal depolarization, we can provisionally refer to such reporter detection as an excitation history.
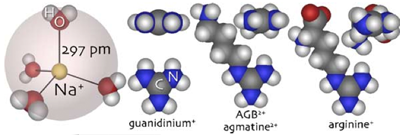
Fig. 1. Comparative sizes of guanidines and solvated Na+ ions. We assume a Pauling ionic radius of 97 pm for Na, a tetrahedrally bonded shell of four water molecules with Na-O bond lengths of 297 pm and H-O lengths of 99 pm. The series of guanidinium, 1-amino-4-guanidobutane (agmatine or AGB) and L-2-amino-5-guanidino-pentanoate (arginine) represents permeant species of increasing dimensions, but all are smaller or similar to the solvated Na ion in size. Each cation is shown in its largest and smallest rotational profile. Molecule shapes were generate as SMILES strings (see Weininger et al., 1989; also see Table 1 and https://en.wikipedia.org/wiki/Simplified_Molecular_Input_Line_Entry_Specification), translated to Brookhaven Protein Data Bank *.pdb format using the CORINA resource (https://www.molecular-networks.com/software/corina/index.html) and rendered with iMol v0.30
References
Balasubramanian, S., Lynch, J. W., & Barry, P. H. (1995). The permeation of organic cations through cAMP-gated channels in mammalian olfactory receptor neurons. Journal of Membrane Biology 146 , 177-191.
Datta, S. N., & Iyengar, S. S. (1991). Semi-empirical Hartree-Fock calculations on the mechanism of enniatin B mediated transport of sodium ions. Chemistry and Physics Letters, 183, 491-498.
Dwyer, T. M., Adams, D. J., & Hille, B. (1980). The permeability of the endplate channel to organic cations in frog muscle. Journal of General Physiology, 75, 469-492.
Fairbanks, C. A., Schreiber, K. L., Brewer, K. L., Yu, C.-G., Stone, L. S., Kitto, K. F., Nguyen, H. O., Grocholski, B. M., Shoeman, D. W., Kehl, L. J., & Regunathan, S. (2000). Agmatine reverses pain induced by inflammation, neuropathy, and spinal cord injury. Proceedings of the National Academy of Sciences of the United States of America, 97, 10584-10589.
Larramendi, L. M., Lorente de No, R., & Vidal, F. (1956). Restoration of sodium-deficient frog nerve fibres by an isotonic solution of guanidinium chloride. Nature, 178, 316-317.
Marc, R. E. (1999b). Mapping glutamatergic drive in the vertebrate retina with a channel-permeant organic cation. Journal of Comparative Neurology, 407(1), 47-64.
Raasch, W., Schafer, U., Chun, J., & Dominiak, P. (2001). Biological significance of agmatine, an endogenous ligand at imidazoline binding sites. British Journal of Pharmacology, 133, 755-780.
Tasaki, I., Watanabe, A., & Singer, I. (1966). Excitability of squid giant axons in the absence of univalent cations in the external medium. Proceedings of the National Academy of Sciences of the United States of America, 56, 1116-1122.
Weininger, D., Weininger, A., & J.L., W. (1989). SMILES. 2. Algorithm for generation of unique SMILES notation. Journal of Chemical Information and Computer Science, 29, 97-101.
Marclab Papers using AGB as a glutamate-gated channel probe
Marc RE, BW Jones, JR Anderson, K Kinard, DW Marshak, JH Wilson, TG Wensel, RJ Lucas 2007 Neural reprogramming in retinal degenerations. Invest Ophthalmol Vis Sci 48: 3364-3371. PubMed
Marc RE, M Kalloniatis, and BW Jones. 2005 Excitation mapping with the organic cation AGB2+. Vision Research, 45: 3454-3468. PubMed
Rohrer B, R Blanco, RE Marc, MB Lloyd, D Bok, DM Schneeweis, LF Reichardt 2004 Functionally intact glutamate-mediated signaling in bipolar-cells of the Trkb knockout mouse retina. Visual Neurosci 21:703-13. PubMed
Marc RE and BW Jones 2002 Molecular phenotyping of retinal ganglion cells. J Neurosci 22:413-427. PubMed
Marc RE 1999 Kainate activation of horizontal, bipolar, amacrine and ganglion cells in the rabbit retina. J Comp Neurol 407:65-76. PubMed
Marc RE 1999 Mapping glutamatergic drive in the vertebrate retina with a channel permeant organic cation. J Comp Neurol 407:47-64. PubMed